Agarwood leaf
機能性表示食品
~ 便秘気味の方の便通を改善する ~
Born from an industry-government-academia joint project
Only one functional food material with patent income
ginkgo leaves
(Patent No. 5187802 Laxative and food containing the same)
便通改善時の副作用が極めて少ない弊社独自原料。
In addition to improving bowel movements, detox effect and
The effect of improving the intestinal environment has also been confirmed.

A very rare tree called "God's Tree", cultivated without pesticides for more than 8 years.

Scientific name: Aquilaria subintegra
■ Production areas: tropical and subtropical countries such as Thailand, Vietnam, and Cambodia
■ The agar wood of the wood partThe king of fragrant trees "Kami no Ki"It is called such as fragrant wood and resin.
The resulting fragrance oil is distributed at a high price. (It is also used in herbal medicine.)
■ History: Agarwood is recorded in the Nihonshoki.Named by Prince Shotoku.
■ Food experience of jinkou leaves: In Southeast Asia such as Thailand,“Agarwood leaves improve bowel movements.
do goodThere is a traditional saying that
Basic information
Ginkgo leaf extract powder (Food material with functional claims)
Origin: Jinkouha
Effectiveness: bowel movement improvement (Compatible with functional labeling), improvement of intestinal environment, detox
Functional Ingredients: Genquanin 5-O-β-primeveroside, Mangiferin
Properties: powder (light brown to brown)
Recommended daily intake: 300 mg to 600 mg, 600 mg as a food with function claims
Compatible dosage forms: tablets, hard capsules, soft capsules, granules, drinks, jelly, etc.

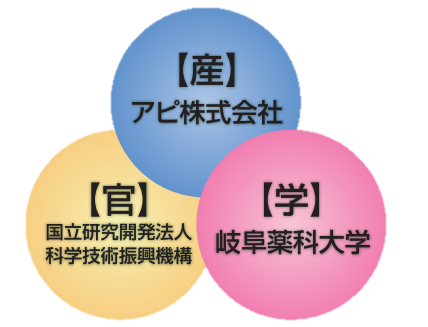
Development history
Commercialization of seeds owned by Gifu Pharmaceutical University
In 2007 (Heisei 19), it was adopted as a 'JST Creative Seeds Deployment Business Consignment Development', and an industry-government-academia collaborative development project started.
After 4 years of research, he succeeded in developing a functional food that relieves constipation.
It was judged that there was a prospect of satisfying the technical requirements of "obtaining labeling permission for food for specified health use", which is the criteria for success or failure.
Carefully cultivated and harvested at reliable contracted farms
I actually went to the site and visited the farm. The raw materials we handle are safe and secure raw materials cultivated and harvested from pesticide-free farms that we can trust.

Effect/Efficacy
Three major effects: improvement of intestinal environment, improvement of bowel movement, and detoxification!
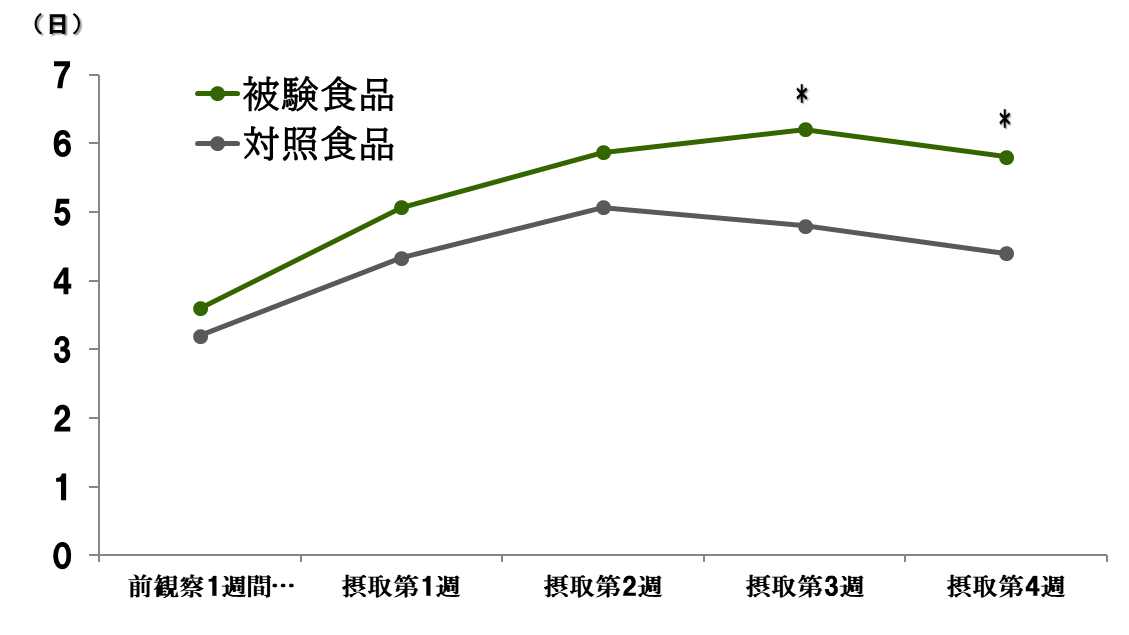
As a result of a clinical trial targeting constipation-prone adult men and women, in two reports that met the eligibility criteria, the amount of defecation, the number of defecations, and the number of defecation days significantly increased, and the quality (hardness) of stools significantly improved. A bowel movement improvement effect was recognized.
In addition, it has been suggested that jinkou leaves may reduce intestinal putrefactive substances (indole) and improve the intestinal environment. It was also confirmed that there was no
A new material that improves bowel movements without strong side effectsis.
While laxatives directly stimulate the large intestine, ginkgo biloba works on acetylcholine receptors and stimulates the small intestine to promote peristalsis, so side effects seen in laxatives (irritant) are extremely rare. can be taken continuously.

evidence
~Functionality~
Non-clinical studies
Improvement of constipation and promotion of defecation
-Action on drug-induced constipation model mice-
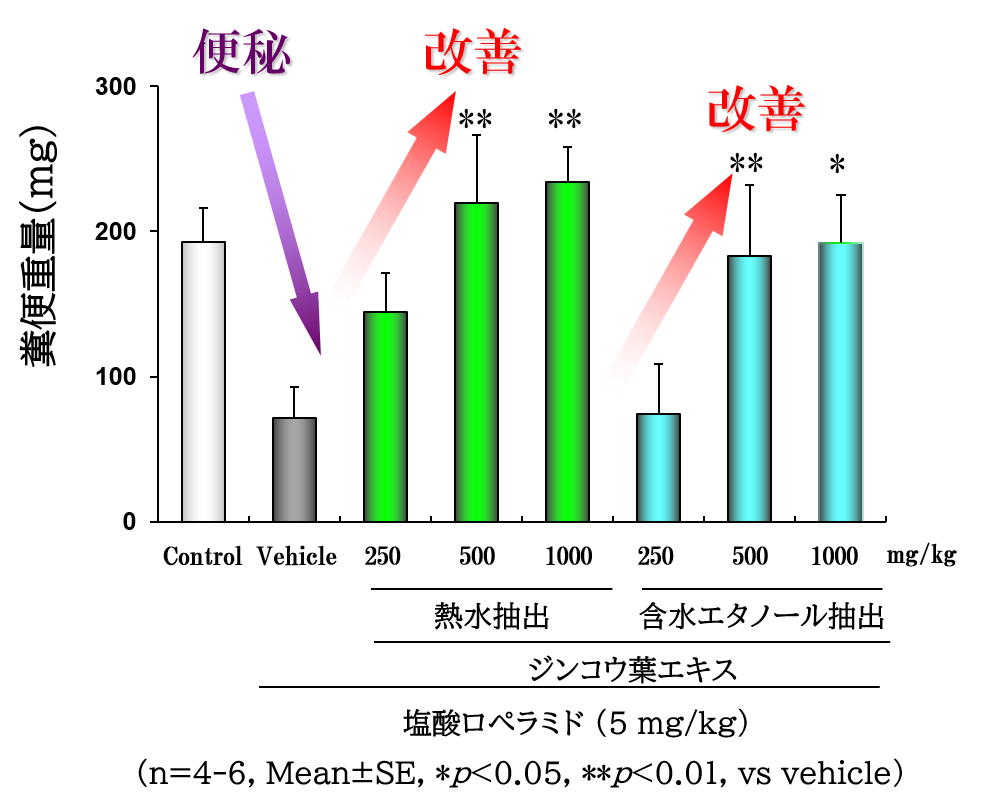
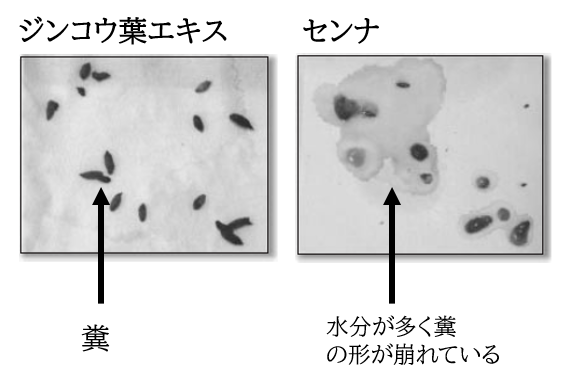
~Comparison of drugs in constipation model mice~
Human clinical trial
Improvement of constipation and promotion of defecation
~600mg/day~
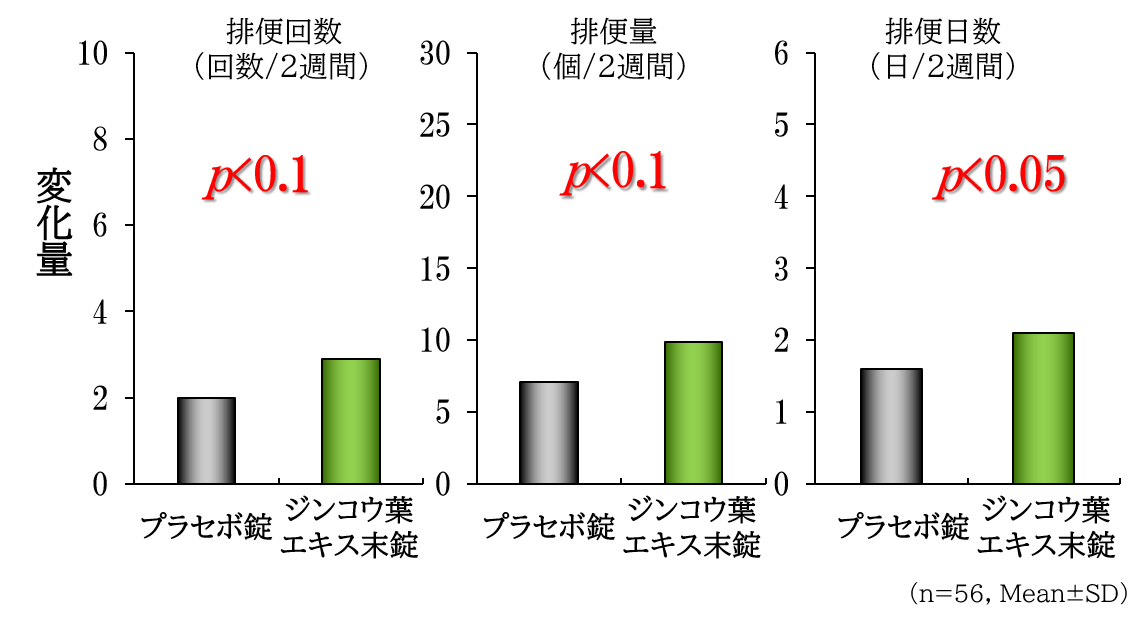
~Defecation days in a week~
evidence
~Safety~
Non-clinical studies
■ Chromosome aberration test
■ Micronucleus test
■ Single dose toxicity test
Repeated dose toxicity test (28 days)
Human clinical trial
(Subjects: 10 men)
(Test method: Open test)
■ Safety confirmation test for adult men and women by long-term intake
(22 males and females aged 20 to under 65)
(Test method: Open test)
■ Safety confirmation test for adult men and women due to overdose
(22 males and females aged 20 to under 65)
(Test method: Open test)
1) Biosci. Biotechnol. Biochem., 74(8), 1550-1555, 2010
2) Jpn Pharmacol Ther 2017, vol.45 no.1
3) Sakai et al., Jpn Pharmacol Ther (Pharmacology and Therapeutics) 2017, vol.45 no.1 113-120, based on Table 4
Efforts to Foods with Function Claims
Recommended dose of ginko leaf extract powder: 600 mg/day
Amount of functional ingredients: Genquanin 5-O-β-primeveroside 1.1 mg, mangiferin 18.0 mg
good bowel week

Notification number: D672 (research review)
Product name: Kaicho week
Functional component name:
This product contains Genkwanin 5-O-β-primeveroside,
Contains mangiferin.
genquanin 5-O-β-primeveroside,
Mangiferin is recommended for people with constipation.
It has been reported to have the ability to improve bowel movements.
Restrictions: 600 mg or more of ginko leaf extract powder per day.
Drinks, capsules, etc. *Different dosage forms from clinical trials
Notification is possible.
Kaicho Week EX

Notification number: D675 (Clinical study of final product)
Product Name: Kaicho Week EX
Functional component name:
This product contains Genkwanin 5-O-β-primeveroside,
Contains mangiferin.
genquanin 5-O-β-primeveroside,
Mangiferin is recommended for people with constipation.
It has the ability to improvebowel movements.
Limit: 600 mg/day of ginko leaf extract powder.
Only the same tablets as in the clinical trial (cannot be changed).
Download materials / Apply for free samples
Leave it to us to support the submission of food with function claims!
Dedicated team provides total support!
Contact
inquiry
3-7-2 Nihonbashi Honcho, Chuo-ku, Tokyo
MFPR Nihonbashi Honcho Building 5th floor
© copyright 2019 API Co.,Ltd. All Rights Reserved.










